What You Need to Know About BIA-ALCL
12
December, 2018
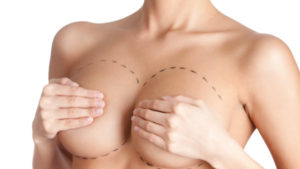
Breast Implants
News
A rare and highly treatable type of lymphoma has recently been highlighted in the news. BIA-ALCL (Breast Implant-Associated Anaplastic Large Cell Lymphoma) came onto the public radar in 2011 when the FDA released a safety communication identifying a possible association between breast implants and the development of BIA-ALCL.
In March 2018, the FDA released another safety communication stating that BIA-ALCL occurs more frequently in implants with textured surfaces. This lymphoma develops around breast implants and scar tissue, not in the breast tissue itself. This is a cancer of the immune system, not a type of breast cancer.

Photo courtesy of plasticsurgery.org
Many women with textured implants are now concerned about what this news means for them. Patient safety is critical to us at Plastic Surgery One. We care about our patients and strive for the highest level of patient care. To that end, we want to provide you with the best information available about this disease. We have created a list of the most commonly asked questions about BIA-ALCL and what the answers are. If you have questions about BIA-ALCL and do not see the information you are seeking below, please contact our office for a consultation.
BIA-ALCL Frequently Asked Questions
Q: What is BIA-ALCL?
A: BIA-ALCL (Breast Implant-Associated Anaplastic Large Cell Lymphoma) is a rare disease that can range from an accumulation of fluids around a breast implant to a potentially serious lymphoma, especially when there are delays in diagnosis. BIA-ALCL is not a cancer of the breast tissue itself. When diagnosed early, it is readily curable. If the disease is advanced, chemotherapy or radiation may be required.
Q. Have there been any deaths due to BIA-ALCL?
A. There have been 16 confirmed deaths, (globally), attributed to BIA-ALCL since the disease. was first reported nearly 20 years ago.
Q: What are the symptoms of BIA-ALCL?
A: The first symptom of BIA-ALCL is usually a swelling of the breast between 2 to 28 years after the insertion of breast implants, with an average of about 8 years after implantation. The swelling is due to a collection of fluid surrounding the implant. This fluid can cause the breast to enlarge significantly over a period of days or weeks. It can also present as a lump in the breast or armpit, firmness of the breast, or pain. It is usually easily and completely treated if patients see their doctor at the first symptom.
Q: What is the risk of developing BIA-ALCL?
A: Based on current data, the risk can be explained by the texture grade of the implants as
- Grade 1 (Smooth only) – The current lifetime risk is zero.
- Grade 2 (e.g. Microtexture, Siltex and similar) – 1:82,000
- Grade 3 (e.g. Macrotexture, Biocell and similar) – 1:3,200
Q: If a breast implant patient sees a plastic surgeon when she develops a first symptom, will she be cured?
A: Most of the time patients see their plastic surgeon right away when they develop significant swelling of the breast. In these cases, the disease is almost always caught early and cured with a straightforward operation. Some women with advanced disease ignored earlier symptoms or saw a doctor who did not properly diagnose them. There are a few patients who presented with advanced disease who said that they never had earlier symptoms.
Q: Is it a problem with Saline or Silicone implants?
A: Of the 414 reported cases of BIA-ALCL, 312 reports included information on the types of implants used. Of those,234 reported implants with silicone gel and 119 reported implants filled with saline. It appears to purely be related to the surface of the implant and not to what the implant is filled with.
Q: How does this impact those with breast implants?
A: ASAPS and ASERF emphasize that the most important issue for women with breast implants is to screen for breast cancer with self-exam, a regular physician exam, and mammography/ultrasound/MRI as recommended by their physician. Regardless of BIA-ALCL, all women should see their plastic surgeon immediately if they note a change to the size, feel, or shape of their breasts.
Q: What about those considering breast implants?
A: BIA-ALCL is only associated with textured surface implants so this is not relevant to patients considering smooth-walled breast implants. But patients considering textured breast implants should discuss this issue with their plastic surgeon. Since our knowledge of this condition is continuing to evolve, thanks in large part to ASERF-sponsored research, patients should check surgery.org and the FDA website for any updates.
Q: What if a doctor is recommending textured implants to a patient?
A: The choice of implant type is ultimately a decision between an educated patient and her board-certified plastic surgeon. There may be certain circumstances where a textured implant is recommended for particular patients.
Q: How is BIA-ALCL diagnosed?
A: If a woman develops swelling in an augmented breast, she should undergo an ultrasound scan. If fluid is detected, it should be drained and tested for cytology. The majority of seromas seen clinically are benign seromas and not BIA-ALCL.
If a patient wants to have their textured implants removed and replaced, the options are:
- Exchange to smooth implants with or without capsulectomy.
Q: How is BIA-ALCL treated and what is the prognosis?
A: Current recommendations for the treatment of BIA-ALCL call for bilateral capsulectomy (removing all the scar tissue) and removal of the old breast implants. This is a very common procedure performed by plastic surgeons, Smooth implants can be put back in or the patient can choose not to have implants. In all early-stage cases, the disease has been fully resolved by this surgery alone. The majority of patients require no additional treatment. However, if the disease has spread to lymph nodes or grown into the adjacent tissues, chemotherapy and radiation may be necessary. These are very serious treatments with significant side effects.
Q: Should patients have their implants removed because of BIA-ALCL?
A: Since BIA-ALCL has only been found with textured breast implants, smooth implant patients do not need to be concerned. For textured implant patients, neither the FDA nor any plastic surgery society currently recommends that women should preventatively remove textured breast implants to prevent BIA-ALCL. Breast implant patients should have ongoing follow up. Current FDA recommendations and ASAPS recommendations indicate that patients with textured implants with no issues should not do anything and implant removal is not recommended.
Q: Should women with breast implants be screened for BIA-ALCL?
A: There is no blood test to specifically screen for BIA-ALCL.
Q: What causes BIA-ALCL?
A: The best theory today is that a combination of four factors are required for the development of BIA-ALCL:
- Textured implants
- Chronic bacterial-inflammation
- Genetic predisposition
- Time
Q: Does ASAPS recommend against the use of textured implants?
A: The available data does not support discontinuance of textured implants. The best practice is always for the physician to discuss with each patient the known risks and potential complications associated with any procedure. It is important for the patient and her doctor to frankly discuss all options available, and the risks involved.
Every plastic surgeon offers patients options regarding breast implants in terms of sizing, shape, and surface. Depending on a particular patient’s needs, a textured implant may be preferable. The plastic surgeon must provide a frank and transparent discussion regarding the benefits and risks of implants, both smooth and textured. The patient must then make an informed decision, based upon her own assessment of her needs and the risks involved.
Every plastic surgeon needs to help each individual patient make her own decision about which implant she prefers in a fully transparent manner. This involves weighing any possible increased risks against the advantages offered by a particular type of implant. It is critical that the patient makes a fully informed decision following a full discussion of the risks and benefits.
For additional information, please refer to https://www.surgery.org/media/resources, or contact our office using the form below for a consultation.
A New You Awaits
Flexible appointments are available.
Or call — 301-245-1829
PSO Request an Appointment
You...Only Better
Contact us today for your free, no-obligation consultation.